 Welcome to Samantha Dicker, the latest guest in our FSHN Research Journeys series, which follows graduate students’ research in the Food Science and Human Nutrition program at The University of Florida. Samantha is a food science master’s degree student, and she aims to understand how human norovirus may survive in cooked oysters. Through her essential research into the impact of organics on human norovirus survival, she strengthens her dedication to food safety research and ensures every oyster is prepared safely and deliciously.
Welcome to Samantha Dicker, the latest guest in our FSHN Research Journeys series, which follows graduate students’ research in the Food Science and Human Nutrition program at The University of Florida. Samantha is a food science master’s degree student, and she aims to understand how human norovirus may survive in cooked oysters. Through her essential research into the impact of organics on human norovirus survival, she strengthens her dedication to food safety research and ensures every oyster is prepared safely and deliciously.
Samantha: Human norovirus is the leading cause of foodborne illness within the U.S., with raw bivalve mollusks, such as oysters, serving as a common source of human norovirus-associated foodborne illness.1,2 However, human norovirus infections from cooked oyster products have raised concerns regarding the efficacy of the recommended cooking guidelines established by the Centers for Disease Control and Prevention (CDC).3
The Persistence of HuNoV in Oyster Tissues
As the global demand for seafood has grown, aquaculture production has increased and now supplies more than 50% of the global supply of fish.4 As of 2016, mollusks (such as oysters) accounted for 21% of seafood production worldwide.4 At the same time, bivalve mollusks are a common food product associated with human norovirus transmission.2
Oysters are the leading mollusk species produced and are commonly associated with human norovirus foodborne outbreaks when consumed raw or cooked. The CDC recommends cooking oysters to an internal temperature of at least 145°F (62.8°C).5 However, the National Outbreak Reporting System reported that from 2010 to 2020, approximately 8% of oyster-associated norovirus outbreaks can be attributed to cooked oysters (i.e., steamed, fried, baked).6 This fact raises concerns regarding the established cooking methods’ ability to inactivate human norovirus or prevent this virus from causing infection.4 Human norovirus may “survive” cooking temperatures as it is often embedded within the tissues of oysters. Therefore, from our research, we aim to better understand how oyster tissues might impact the longevity of human norovirus when undergoing heat treatments.

By understanding how cooking impacts the survival of human norovirus in oysters, we can potentially decrease the number of foodborne illness cases and their associated public health concerns. Furthermore, this research will benefit the stakeholders of the oyster production world, as decreasing foodborne outbreaks will help maintain their credibility as safe producers and prevent associated economic losses.
The Turning of a Tide in Samantha’s Academic Journey
I entered the third year of my undergraduate degree at the University of Florida intending to pursue a career as a registered dietitian. However, as I spent more time volunteering in UF’s Field and Fork food pantry and gained more experience in food science coursework, my interest grew in pursuing further education in food safety.
Equipped with nothing more than a limited knowledge of microbiology and even less experience in food safety, I applied for an undergraduate research position in Dr. Naim Montazeri’s food virology laboratory. Since then, I have cultivated a deep-rooted respect and passion for food safety research and developed knowledge regarding our food system and the pathogens present.
The culmination of my experiences within the laboratory and my coursework has shown me the significance of viruses within our food system and the importance of food safety research in ensuring a safe food supply. Human norovirus is the leading cause of foodborne illness worldwide, yet knowledge regarding norovirus survival and interactions within the environment is limited.1 Researchers lack efficient and widely available laboratory techniques to assess viral infectivity or its ability to infect its target (or host) across different conditions.7 The challenges associated with this research motivate me to continue to grow and develop as a researcher and future food safety professional.
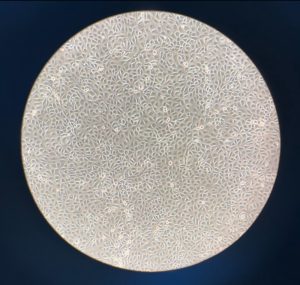
How to Assess the Thermal Inactivation of Human Norovirus in Oyster Tissues
Previous research examining the thermal inactivation of human norovirus within oyster tissues has been conducted using surrogates (e.g., Tulane virus) for human norovirus.8,9 Human norovirus does not have an established method to assess viral infectivity. Therefore, researchers use surrogates or other viruses with similar characteristics or behaviors resulting from examined conditions instead of human norovirus.8,9 Surrogates are also used when studying viruses that are too dangerous to directly study or if researchers cannot obtain the actual virus. While surrogates are carefully selected due to their similarities to the virus being examined, they do not fully represent how this virus will behave in the same conditions.
Our research seeks to address the limitations of previous work by studying both the Tulane virus (as a surrogate) and a human norovirus strain. The tissues will be dissected and separated from multiple oysters and blended into an oyster homogenate, for each of the individual tissue samples (i.e., gills, digestive tract, etc.). These samples will then have a known concentration of virus added to them and placed under varying temperature conditions resembling cooking times and temperatures.
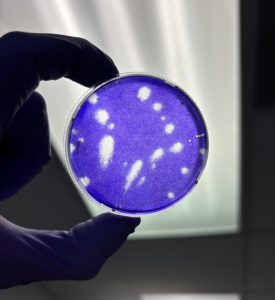
From Testing Oysters to Developing New Risk-based Models
For the samples infected with the Tulane virus, we will conduct an infectivity plaque assay using a host cell, or a cell that the virus will bind to and infect, to determine the amount of infectious virus that remains. Our lab lacks access to host cells for human norovirus to conduct an infectivity assay. Therefore, reverse transcription polymerase chain reaction (RT-qPCR) will help quantify the viral genome present after the heat is applied for both human norovirus and Tulane virus.
This research project is a subset of two others that will be conducted to study the risk of norovirus infection when consuming cooked oysters. My research will investigate the persistence of human norovirus in different oyster tissues by applying cooking temperatures and times. Additional research conducted by fellow graduate students will further expand the investigation of the thermal inactivation of human norovirus to the entire oyster in a similar method used for the tissues. These data will be used to establish risk-based assessment models that will be used to measure the risk posed by consuming cooked oysters in different scenarios (i.e., how much virus is present before cooking, the cooking method used, and so on).

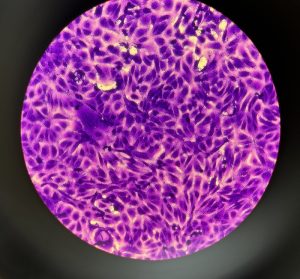
The Challenges Posed from Virus Research
Due to a lack of an available infectivity assessment for human norovirus, we will perform RT-qPCR. This technique will provide a quantification of the total virus genome present rather than just the infectious viral particles, thus potentially resulting in an overestimation of viral survival and consumption risk.10
To combat overestimation, we are implementing techniques that allow a more accurate measurement of only infectious viral particles within the sample. We plan on using the surrogate, Tulane virus, as an infectivity assay and RT-qPCR with a viability pretreatment can be used to measure the impact of heat on Tulane virus inactivation to better determine the most effective temperature and time combination of preparing safe oysters. The RT-qPCR viability pretreatment technique developed will also be used for heat-treated human norovirus to better measure the viral persistence and more accurately predict the risk posed by consuming that oyster product.
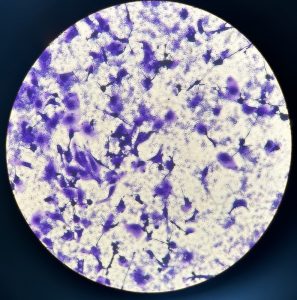
While overcooking oysters may seem like a potential solution to avoid norovirus, it may result in an undesirable tough and chewy texture. Therefore, our research seeks to mitigate the risk associated with cooked oysters while maintaining the delicate experience provided by their consumption.
Samantha is a food science master’s degree student working in Dr. Naim Montazeri’s Food and Environmental Virology Laboratory. She is a double Gator and is excited to further represent the Gator Nation and contribute to the novel field of food virology to ensure the safety and security of the global food supply chain.
References
- Burden of Norovirus Illness in the U.S. | CDC. Published January 12, 2022. Accessed November 27, 2022. https://www.cdc.gov/norovirus/trends-outbreaks/burden-US.html
- Pouillot R, Smith M, Van Doren JM, et al. Risk Assessment of Norovirus Illness from Consumption of Raw Oysters in the United States and in Canada. Risk Anal. 2022;42(2):344-369. doi:10.1111/risa.13755
- Alfano-Sobsey E, Sweat D, Hall A, et al. Norovirus outbreak associated with undercooked oysters and secondary household transmission. Epidemiology & Infection. 2012;140(2):276-282. doi:10.1017/S0950268811000665
- Botta R, Asche F, Borsum JS, Camp EV. A review of global oyster aquaculture production and consumption. Marine Policy. 2020;117:103952. doi:10.1016/j.marpol.2020.103952
- CDC. Foods that can cause food poisoning, Accessed 11/9/2023, https://www.cdc.gov/foodsafety/foods-linked-illness.html. Centers for Disease Control and Prevention. Published August 9, 2023. Accessed November 9, 2023. https://www.cdc.gov/foodsafety/foods-linked-illness.html
- National Outbreak Reporting System (NORS) Dashboard. Centers for Disease Control and Prevention (CDC). Accessed September 17, 2023. https://wwwn.cdc.gov/norsdashboard/
- Costantini V, Morantz EK, Browne H, et al. Human Norovirus Replication in Human Intestinal Enteroids as Model to Evaluate Virus Inactivation. Emerg Infect Dis. 2018;24(8):1453-1464. doi:10.3201/eid2408.180126
- Shao L, Chen H, Hicks D, Wu C. Thermal inactivation of human norovirus surrogates in oyster homogenate. International Journal of Food Microbiology. 2018;281:47-53. doi:10.1016/j.ijfoodmicro.2018.05.013
- Araud E, DiCaprio E, Ma Y, et al. Thermal Inactivation of Enteric Viruses and Bioaccumulation of Enteric Foodborne Viruses in Live Oysters (Crassostrea virginica). Appl Environ Microbiol. 2016;82(7):2086-2099. doi:10.1128/AEM.03573-15
- Shan L, Yang D, Wang D, Tian P. Comparison of cell-based and PCR-based assays as methods for measuring infectivity of Tulane virus. Journal of Virological Methods. 2016;231:1-7. doi:10.1016/j.jviromet.2016.01.012
Looking for more posts exploring graduate research projects in the FSHN Department at the University of Florida?
Dive into the Research Journeys of other graduate students below.
M.S. Food Science
M.S. Nutritional Sciences
Ph.D. Food Science
Ph.D. Nutritional Sciences
 4
4
