 Welcome to FSHN Research Journeys, which follows the research of graduate students in the Food Science and Human Nutrition program at UF. Today’s guest poster is Tamanna Afrin Prami, a first-year doctoral degree student in nutritional sciences. She is studying in the lab of Dr. Mitchell Knutson. Learn about her research into understanding iron overload as well as a possible treatment for its devastating effects on the human body.
Welcome to FSHN Research Journeys, which follows the research of graduate students in the Food Science and Human Nutrition program at UF. Today’s guest poster is Tamanna Afrin Prami, a first-year doctoral degree student in nutritional sciences. She is studying in the lab of Dr. Mitchell Knutson. Learn about her research into understanding iron overload as well as a possible treatment for its devastating effects on the human body.
Tamanna:
In November 2018, BBC Health reported a case with mystifying symptoms. Katherine Hough, 27, suffered for four years with unexplained stomach pains in addition to severe joint pains, hair loss, and fatigue. After careful investigation and blood tests, she was diagnosed with hereditary hemochromatosis, a genetic condition that causes iron overload in the body.
The Dangers of Iron Overload
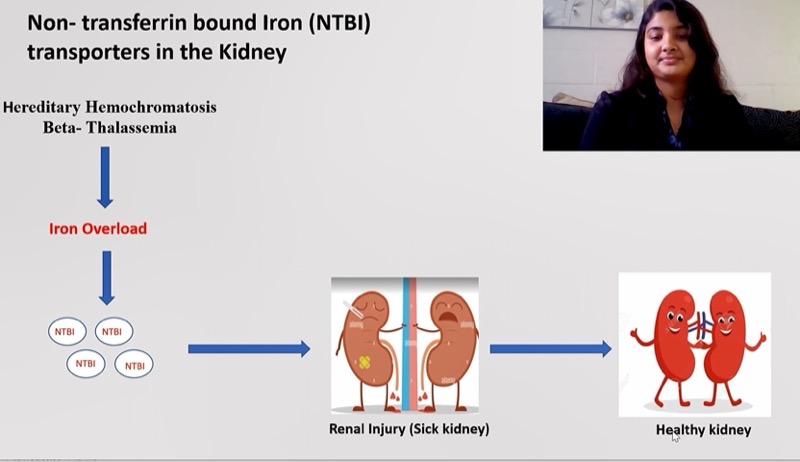
Iron overload is seen in hereditary hemochromatosis and beta-thalassemia. Approximately, one million people in the US suffer from hereditary hemochromatosis. About 0.4% of the US population and 1.5% 0f the global population are carriers of beta-thalassemia.1 But how does iron overload happen?
In the human body, iron transporters take up iron into cells and tissues. Under normal conditions, cells get iron from transferrin, an iron transport protein in blood plasma. Iron in blood plasma is tightly bound to transferrin, and transferrin is taken up by cells via the transferrin receptor. Iron overload conditions occur when the level of iron in blood plasma exceeds the carrying capacity of transferrin. In other words, all the transferrin is bound with iron, and the extra unbound iron (non-transferrin bound iron, or NTBI) is taken up into tissues, causing iron accumulation and toxicity.
Abnormal Iron Uptake May Cause Serious Disease

Studying the NTBI uptake process is essential to understanding how iron overload happens. Some cell types and tissues can take up NTBI via membrane transport proteins. So far, only three mammalian iron transport proteins have been identified: DMT1, ZIP14, and ZIP8. Recently, researchers have recognized that ZIP14 and ZIP8 play an important role in the uptake of NTBI during iron overload conditions.2
All three transport proteins are expressed in tissues throughout the body, but their abundances differ among tissues. For example, ZIP14 is highly abundant in the jejunum, liver, pancreas, and heart while ZIP8 is expressed mainly in the kidney, lung, and placenta. During iron overload, these transport proteins can take up iron in their corresponding organs, resulting in toxicity.
This toxicity may lead to various complications including endocrine disorder, acute and chronic kidney diseases, liver cirrhosis, and heart failure. The symptoms that arise from these disorders often include fatigue, lethargy, abnormal heart rhythm, and arthritis. Although iron chelation therapy decreases iron loading, it does not completely prevent it. As a result, patients ultimately develop iron overload and iron-related diseases. Those suffering from these diseases need alternative or additional treatments.
Tamanna’s Path to Study Disorders of Iron Metabolism
I was born and raised in Bangladesh, a developing country that faces many global health issues including malnutrition and nutrition-related chronic diseases. I became interested in my research project while taking a human nutrition course as an undergraduate student. As a part of my coursework, I collected surveillance data on patients with anemia in Bangladesh.
I found that disorders of iron metabolism are very common in my country. Apart from iron deficiency anemia, iron overload disorders are also recognized as a public health concern. These health issues sparked my interest in understanding the transport, metabolism, and homeostasis of iron as well as the development of therapies to treat disorders of iron metabolism.
After completing my undergraduate degree, I searched for nutritional sciences graduate programs. On the UF FSHN Department website, I found that Dr. Mitchell Knutson’s lab focuses on identifying and characterizing iron transport proteins at the molecular, cellular, and physiologic levels. My graduate research project is part of the major aim of the Knutson Laboratory, which is to identify iron transport proteins in various tissues. My graduate research project completely fits my interest in iron metabolism.
Understanding Membrane Transport Proteins is Key to Prevent Iron Overload
Researchers and students in the Knutson Laboratory study the roles of ZIP14 and ZIP8, mainly in the context of iron overload. So far, researchers in our lab have shown that ZIP14 is required for iron uptake into the liver and pancreas during iron overload.3 So, ZIP14 can be a potential therapeutic target in the treatment of iron overload in these organs.
The role of ZIP14 and ZIP8 in the kidney remains unknown. In my research project, I will look specifically at the roles of ZIP14 and ZIP8 in the kidney. My goal is to determine whether ZIP14 and/or ZIP8 are required for iron uptake by the kidney during iron overload. I will use a variety of conditional knockout mouse models to better understand the iron transport system. Lab techniques include western blotting, immunofluorescence, QRT-PCR, and Gamma counting. I will then analyze the data with various statistical tests including the t-test.
Looking to Future Treatments for Iron Overload

My research project is important because the kidney plays an essential role in iron metabolism by reabsorbing iron while filtering urine. Moreover, disturbances in iron balance are recognized as a cause of kidney injury. But we do not yet understand the molecular mechanism of iron handling in the kidney.
A better understanding of iron transport will help us to understand how transporters play a role in kidney disorders such as chronic kidney disease. This research will help to identify therapeutic inhibitors that would increase urinary iron excretion. As a result, we could lessen iron overload-related complications among patients who are suffering from hereditary hemochromatosis like Katherine Hough.
Tamanna Afrin Prami grew up in Bangladesh and completed her B.Sc. in Biochemistry and Molecular Biology at the University of Dhaka, Bangladesh. Now, she is a first-year Nutritional Sciences Ph.D. student in the Department of Food Science and Human Nutrition at the University of Florida.
References
- https://www.centerfornurses.com/beta-thalassemia/what-is-beta-thalassemia/b-thalassemia-epidemiology/
- Mitchell D.Knutson (2019). Non-transferrin-bound iron transporters. Free Radical Biology and Medicine, Vol 133.
- Supak, W. C. (2015). SLC39A14 Is Required for the Development of Hepatocellular Iron Overload in Murine Models of Hereditary Hemochromatosis. cell metabolism, 27.
 2
2
