Article by: Jeffrey S. Block, M.D., Physician Anesthesiologist, UF/IFAS Industrial Hemp Pilot Project Advisory Committee, UF/IFAS Master Gardener Miami-Dade County
Correspondence to: 7299 SW 79 Court – Miami, FL 33143, DocBlock@BellSouth.net
Edited by: Zack Brym
This insights article is part of the blog series Perspectives from the Hemp Industry.
Cannabis and Human Origins
In ancient times, healers were facultative botanists with an intimate local knowledge of remedies. A special craft of healers was to keep valuable plants alive for their bag of remedies. From the earliest of primitive nomadic tribes, to the advancing agricultural civilizations that followed, botany remained central to mankind’s evolutionary success. Early civilization’s “physician” healers included Imhotep (2667-2648 BC) in Egypt and Hippocrates (460-370 BC) in Greece, who understood the importance of plants to sustain health. These two historical figures are widely regarded as Fathers of Modern Medicine in that they recorded their botanical findings to herald the beginning of the evidence-based healthcare science (Butrica, 2002). A representative of traditional botanical medicine, cannabis has an extraordinarily long history, with its oldest documentation appearing in Chinese medicine dated to circa 2700 B.C. The long history between cannabis and people could date back to the origins of agriculture with lasting impacts on agriculture and human physiology (Clarke and Merlin, 2016).
It may be that over the past 10,000 years, association with cannabis interplayed favorably with humans to meet the survival needs and evolutionary adaptation. The origins and global distribution of cannabis is linked to the spread of agricultural settlements from central Asia around the world. Considering the stem, seed, and oil production of cannabis; and mankind’s successful survival alongside, a deeper association, such as a therapeutic tolerance, could evolve. More broadly, evolution through natural selection would reward effective use of botanical substances through benefits to health and well-being. Co-evolution follows that cannabis evolved a human-balanced set of oils. Whether for nutrition, remedies, or ritual, plant-based chemicals are interwoven to human life.
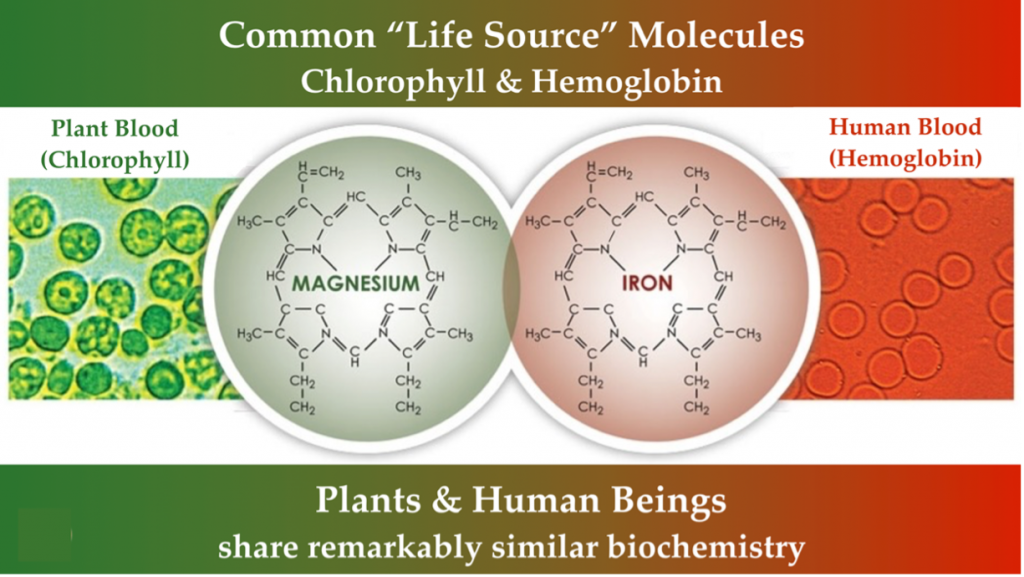
Animal and plant associations are reflected in common chemicals essential to our health and survival. Part of that association is possible because of similarities in life’s core functions. The essential “life blood” molecules of chlorophyll and hemoglobin share molecular similarities linking the evolution of plants and animals. Light energy is processed in plants through chlorophyll, while energy is derived through oxygen exchange in animals through hemoglobin. Chlorophyll and hemoglobin have a similar molecular structure differentiated by a magnesium or iron substitution.
Colonial Quackery and Prohibition
Cannabis reached western medicine’s physicians in the mid-nineteenth century through the French who observed its use in Egypt, and by the British in colonialized India, who chronicled Ayurvedic medicine. Between 1840 and 1900, upwards of 100 articles about the medical use of cannabis were published in western medical journals, primarily touting its utility as an analgesic and antispasmodic. Cannabis was added to the United States Pharmacopoeia in 1852, where it would remain for 90 years until federal laws made production of cannabis medicines prohibitively expensive. During that era, most major pharmaceutical companies provided multiple cannabis formulations, often combined with other substances. As that was also the era of bunk remedies and medical quackery, skepticism about the therapeutic utility of cannabis would have been warranted, but concern for unfounded claims was not the basis for restricting it.
The first federal law in the United States aimed at criminalizing ‘marihuana’ was enacted in 1937 with little debate or acknowledgement of its potential therapeutic properties. The sole exception was the testimony of Dr. William C. Woodward, the legislative counsel of the American Medical Association (AMA), who warned that prohibiting it “loses sight of the fact that future investigation may show that there are substantial medical uses for cannabis” (US Congress, 1937). He announced his opposition to the bill and sought to dispel any impression that either the AMA or enlightened medical opinions sponsored this legislation. Indeed, the tax act and subsequent Controlled Substances Act of 1970 classifying ‘marijuana’ as a Schedule 1 substance also limited agriculture and medical research in the United States.
Cannabis Ethnobotany
Observations of how plants affect pain have helped identify three distinct endogenous receptor systems. These three receptor systems have complementary pathways that may collectively help treat pain more safely and effectively when used in a balanced therapeutic manner. Several decades before the discovery of any of these receptors’ importance to pain perception, major pharmaceutical companies had already combined three plant substances for use as an elixir.

containing combinations of plant-sourced chemicals were common in medicines.
The discovery process of identifying active plant compounds in remedies and their specific interaction within the body has proven fruitful in many instances, although compiling the supporting evidence can often be time and cost consuming. Derived from the bark of willow trees, aspirin’s analgesic properties were commercially known for nearly a century before the discovery of prostaglandins. The opium poppy flower’s analgesic effects were known for thousands of years before endorphins and enkephalins were identified. In 1974, endorphins were discovered through plant-based phyto-opioids derived from the opium poppy. More recently in the 1990’s, endovanilloids were identified through the phyto-vanilloid apsaisin found in the chili pepper; and endocannabinoids were discovered through the study of phyto-cannabinoids produced by the cannabis plant.
Cannabis remains uniquely challenging to study with extra regulatory burdens and limited supply of research materials. Despite those barriers, cannabinoid researchers elsewhere in the world have shared substantial successes investigating both the endogenous system and the action of cannabis plant-derived molecules on it (NASEM, 2017). Additionally, the science of genetics vastly improves our ability to understand mechanisms in evolution and physiology. DNA-based research can help select plants that express desired oil chemistry for potential medicinal use.
Medical Cannabis Standards
Today, as a result of social media and other informal information sharing, many patients hear anecdotal reports suggesting cannabis may help or even cure a remarkably diverse range of conditions. Hyperbole is rampant in these accounts, particularly regarding curative potential, and even knowledgeable patients can hold mistaken beliefs about medical cannabis (Block, 2020). A dearth of clinical research has led to a reliance on anecdotal reports, but the Institutes of Medicine (Joy et al., 1999) and the National Academies of Sciences, Engineering, and Medicine (NASEM, 2017) have identified sound clinical studies supporting the anecdotal and preclinical indications for medicinal cannabis use. Pre-clinical studies often provide the basis for robust results from clinical investigations. Perhaps nowhere has this been more evident than for plant-based remedies in treating pain, which has been far and away the subject of the greatest number of clinical trials.
A clinician faced with their patient in intolerable pain has an obligation to do what is possible to alleviate that suffering without causing harm. As the limitations of opioid options become more evident, the search for safe and effective solutions has rightfully included cannabis remedies and further derived medicines. The human endocannabinoid system is remarkably complicated and is further complicated by the interaction with plant-based phytocannabinoids appearing in cannabis remedies. When delivered to the body, phytocannabinoids demonstrate non-selective action for longer time periods and more broadly across the body than the endocannabinoids produced within the body. Acknowledging potential harm of phytocannabinoids or their delivery method is important with contraindications for patients with existing heart disease, preexisting or family history of psychosis, and pregnancy. Yet, randomized clinical trials provide substantial evidence of the pain-killing potential of cannabinoids that confirm animal experiments, laboratory science, and what is known about the purpose of the endocannabinoid receptor system.
An initiative is evolving for clinicians to incorporate phytocannabinoids in their medicine bag. Most major medical schools currently offer limited training on pain management and cannabinoids; with bioethical objections leading a charge to increase availability of such education. Instead, training focuses on preparing future licensed practitioners to achieve certain minimum standards along the examination and licensure process. Until physician education standards include knowledge on endogenous cannabinoid signaling and plant-derived cannabinoid medicines, the burden falls on individual clinicians to educate themselves.
In addition to the evidence of 10,000 years with access to phytocannabinoids, a 2013 National Institute of Health summary report envisions that “modulating the endocannabinoid system may have therapeutic potential in almost all diseases affecting humans” (Pacher and Kunos, 2013). This most remarkable statement could reconnect a botanical remedy with the receptor system discovered through its use with extraordinary potential for diverse therapeutic applications.
References
Block, Jeffrey S. 2021. Chapter 4: Endogenous Cannabinoid Receptors and Medical Cannabis. In: Advanced Therapeutics in Pain Medicine. Eds. Sahar Swidan, Matthew Bennett. CRC Press. Boca Raton, FL.
Butrica, James L. 2002.The Medical Use of Cannabis Among the Greeks and Romans.Journal of Cannabis Therapeutics,2:2,51-70. https://doi.org/10.1300/J175v02n02_04
Joy, Janet E., Stanley J. Watson, Jr., and John A. Benson, Jr., Eds. 1999. “Marijuana and Medicine: Assessing the Science Base.” Division of Neuroscience and Behavioral Health, Institute of Medicine,
National Academy Press, Washington, D.C. https://www.nap.edu/read/6376/.
National Academies of Sciences, Engineering, and Medicine. 2017. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press. https://doi.org/10.17226/24625.
Pacher, Pal and George Kunos. 2013. Modulating the endocannabinoid system in human health and disease – successes and failures FEBS Journal; 280(9): 1918–1943. https://doi.org/10.1111/febs.12260.
U.S. Congress. May 4, 1937. House of Representatives Committee on Ways and Means: Taxation of Marihuana Hearing before the Committee on Ways and Means, 75th Cong., 1st sess.
 0
0
