In a recent study, researchers at the UF/IFAS Soil and Water Sciences Department (SWSD) demonstrated that small wastewater treatment plants (WWTPs) can benefit from struvite recovery. This method lowers phosphorus (P) output and conserves an extremely valuable natural resource. It is a sustainable way of keeping P local.
Phosphorus is an essential nutrient for all living things. Phosphorus fertilizer applications are used to maximize the production of food, forage, and fiber. Indeed, there is no substitute for phosphorus in agriculture. However, mineral phosphate fertilizer is a limited natural resource produced by mining phosphate rock. There are concerns that the earth’s phosphate rock reserves may be exhausted within decades. Therefore, it will become increasingly critical for the agriculture industry to identify alternative sources of phosphorus-based fertilizer. At the same time, over-application of phosphorus as soluble fertilizer or from organic sources (i.e., manures, litter, biosolids) can negatively impact water quality. Excess phosphorus runoff leads to eutrophication and subsequent degradation of water bodies. In recent years, phosphorus loads to the world’s rivers have doubled because of fertilizer runoff, sewage discharge, deforestation, and soil erosion. It is essential, therefore, to recycle phosphorus and utilize phosphate-containing wastes as efficiently as possible, while keeping fugitive nutrients out of watersheds and associated surface waters.
WWTPs
In the U.S., there are more than 14,000 municipal wastewater treatment plants processing approximately 0.34 million tons of phosphorus annually or 18% of the total phosphorus consumed in the U.S. Over a third of this wastewater treatment (based upon flow) occurs at small (≤38,000 m3 d−1) operations.
At WWTPs, phosphorus removal occurs mainly through the removal of biosolids. Thus, biosolids are a viable source of plant nutrients for use as fertilizer in agriculture. However, biosolids application rates can often lead to excess phosphorus application relative to crop needs. Also, in Florida, over a third of biosolids end up in landfills, where the nutrient value is essentially lost forever. Capturing wastewater phosphorus through struvite (MgNH4PO4•6H2O) precipitation is an attractive option for phosphorus recovery and re-use in agriculture. Struvite is a ‘clean’ source of nitrogen (N), phosphorus, and magnesium (Mg), and it can be effectively stored and applied in a more targeted manner, much like conventional mineral fertilizer. Thus, struvite recovery provides a potential fertilizer revenue stream for WWTP facilities. Also, by recovering struvite crystals, less phosphorus is exported off-site through effluent or biosolids. This lessens its impact on the environment, particularly landfills and ecologically sensitive water bodies.
Study
The SWSD researchers used a systems dynamic modeling approach and data from three small, rural WWTP facilities in North Florida. This allowed them to assess the potential impact of intentional struvite crystallization recovery on wastewater treatment plant (WWTP) allocation of N and P to effluent and biosolids outputs.
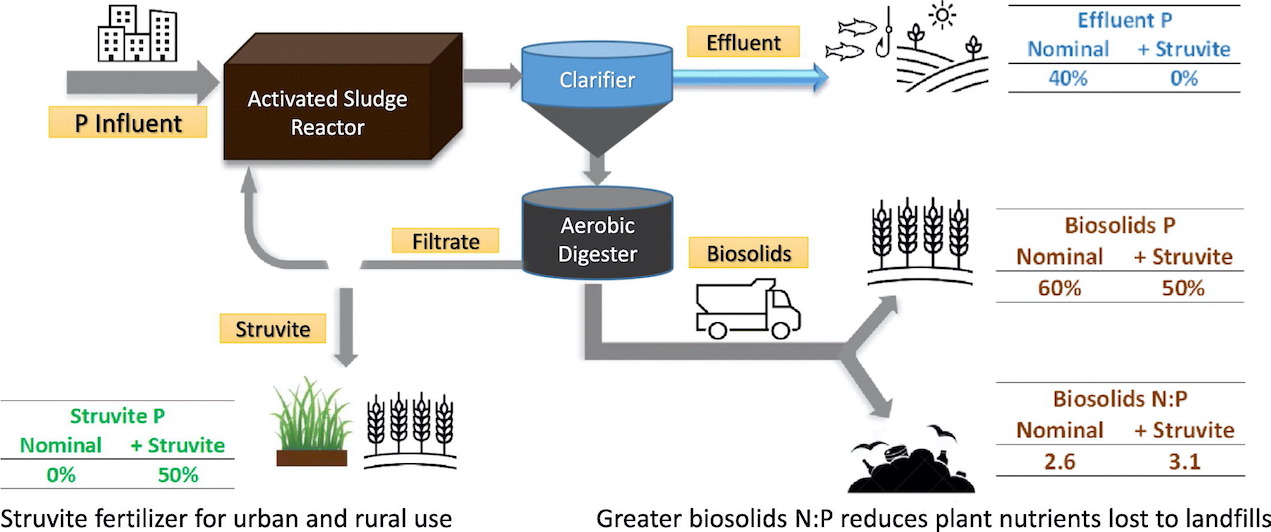
The results show struvite crystallization reduced the amount of P leaving the facilities in effluent by 37 to 100 percent, depending upon the WWTP. In addition, biosolids P reductions ranged from 17 to 46 percent. The model also predicted a 37% average increase in the biosolids N:P ratio. Increasing the N:P ratio may allow for greater biosolids land-application rates where P fertilizer restrictions exist. This nitrogen and phosphorus recovery would divert nutrients essential for plant growth from landfills while increasing the availability of limited landfill space for other materials.
Overall, the team found that incorporating struvite crystallization and recovery into small rural WWTPs reduces effluent post-treatment needs. The crystallization process also results in a more useful biosolids product. You can read the full report on phosphorus recovery here: Mitigating rural WWTP impacts research.
Featured image at top of page from Google Maps © 2020.
 1
1
