When it comes to household drinking water quality, one of the most common concerns from homeowners is hard water.
What is hard water?
Water hardness refers to the amount of calcium and magnesium ions in the water. An ion is a particle that carries a charge. Charged particles are “eager” to reach a neutral state either by gaining or losing electrons, making them very reactive. Hardness ions reacting with carbonate ions in the water, especially combined with heat, can form a chalky deposit called scale (Wilson).
Where does it come from?
These ions are omnipresent in Florida groundwater. To understand why, we need to take a quick detour into Florida geology.
Florida’s landmass started under a shallow sea, inhabited by calcium carbonate-rich organisms like corals, oysters, starfish, mussels, and more. As these organisms lived out their life cycle, their remains were deposited layer by layer on the seafloor. Heavy accumulation of this material eventually formed the Florida peninsula. The last phase of Florida land development delivered the sand, clay, and silty surface materials that we live on today (Bostick et al.).
In most of Marion County, wells are drawing water from within that carbonate landmass. As you can imagine, pumping groundwater that is flowing through calcium carbonate deposits results in some of that material dissolving into solution and ending up in your well.
What does it do to health and home?
Hard water is not bad for us, calcium and magnesium are an essential part of our diet and tend to improve water taste. The issue comes from extremely hard water. If the concentrations are high enough, scale can excessively build on the interior of pipes, inside water heaters, faucets, dishwashers, and other appliances eventually reducing efficiency and lifespan. Additionally, hard water can dry out hair and skin, along with decreasing soap’s ability to lather (Swistock et al.).
Some folks, especially those with metal plumbing, stand to benefit from hard water. Moderately hard water can deposit a thin layer of scale on the inside of your household plumbing to protect against corrosion. This is a common technique called “corrosion inhibition” used in large drinking water municipalities to protect metal pipelines from interior corrosion that can result in excess metals in the drinking water supply.
How to fix?
Water softeners are often used to correct hard water. Hard water from your well is pumped through a sand-like media that is coated with sodium ions that swap places with hardness ions in your water. This process, called “ion exchange” forms sodium carbonate, which is much more water-soluble and will not form a scale in appliances or pipes (Swistock et al.).

Having hard water doesn’t always mean you have to use a water softener. Other than the initial cost of the unit, this process also comes with some disadvantages. Chiefly, the ion exchange resin must be regenerated with positive ions. This process involves flushing the resin with a salt solution. Most systems will do this automatically, though softener salts need to be purchased and refilled regularly, and the flushing process uses a considerable amount of water. In addition, sediment, oxidized metals, bacteria, and fungi can accumulate on the resin and decrease efficiency. If this becomes a reoccurring issue, a water pretreatment system may need to be installed before the softener. Depending on the type of salt used in the system, this process can also introduce extra sodium into your diet (Swistock et al.). Per unit of hardness removed, approximately half of that is replaced by sodium. For example, if the softener removes 50 mg/L of hardness ions, 25 mg/L of sodium will be added.
Soft or not?
The choice of purchasing a water softening unit for your home is less related to health concerns and more about appliance efficiency and scale deposition. If you have extremely hard water over 180 mg/L, certainly consider a water softener to protect your plumbing and appliances. Otherwise, spend some time weighing the pros and cons of a softener versus hard water.
Use the table below for a quick reference:
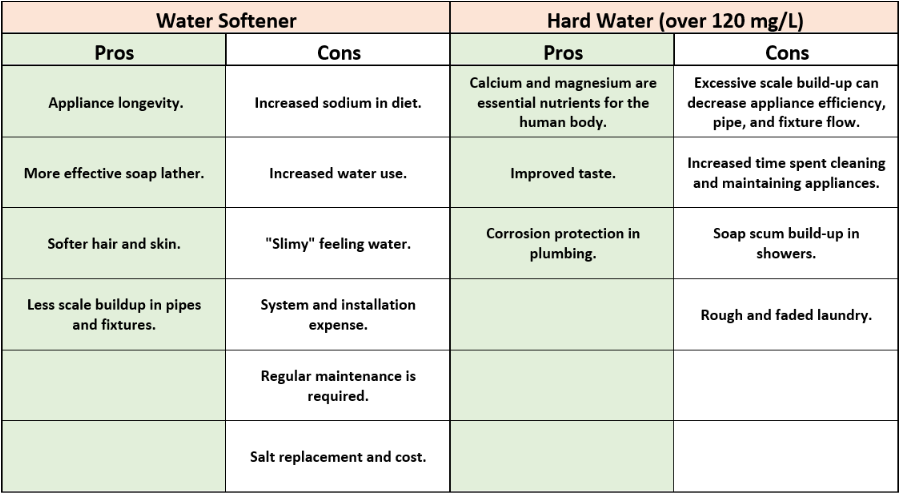
Keep in mind, that many combined factors contribute to scale deposition and even if you have hard water, it’s not always necessary to soften it. There are additional maintenance procedures that can be implemented in your home to reduce the impacts of hard water on your appliances.
These include:
- Regular use of dishwasher cleaners; these include specially formulated ingredients that reduce scale and grease build-up while still protecting gaskets and seals.
- Flushing and cleaning water heater with acid-based cleaners
- Cleaning faucets, aerators, and showerheads with acid-based cleaners
- Running vinegar or lemon juice through coffee maker
A small note on the cleaners mentioned above: Keep a lookout for Environmental Protection Agency (EPA) Safer Choice options. These products are certified by the EPA to be products that contain ingredients that are safer for humans and the environment.
In summary, hard water is more of a nuisance than a health concern. The choice to soften your water is individual preference unless you have excessive levels of hardness. There are many factors to consider during the decision-making process so be sure to reach out to the UF/IFAS Marion County Extension office and work closely with a certified well contractor, water system operator, or trusted treatment specialist.
Sign up for the UF/IFAS Marion County Water Resources Newsletter!
Works Cited
Bostick, Kyle W., et al. “FLORIDA’S GEOLOGICAL HISTORY.” Ask IFAS- Powered by EDIS, University of Florida, 31 Mar. 2019, https://edis.ifas.ufl.edu/publication/UW208.
Swistock, Bryan R., et al. “Water Softening.” Penn State Extension, Penn State Extension, 12 July 2011, https://extension.psu.edu/water-softening.
Wilson, Chris. “WATER QUALITY NOTES: ALKALINITY AND HARDNESS.” Ask IFAS- Powered by EDIS – Powered by EDIS, University of Florida, 17 Sept. 2019, https://edis.ifas.ufl.edu/publication/SS540
 1
1
