There are many chemical compounds that are important for the existence of life on our planet, but many would agree that water is the most important. It is the miracle molecule. It provides a means of getting needed materials into our body, transporting it around the body, and excreting toxins from the body. It helps gives us form – it makes up 80-90% of our bodies. The vast majority of space in a cell is taken up with water. We know how important it is to drink it. Some say eight glasses a day, I try to get at 32 ounces in each day. People have gone as long as 70 days without food, but most cannot make it more than three without water. It is truly an important compound and we are lucky to have a planet covered with it. 70% of the surface is covered with the “miracle molecule” and those portions that are dry ground have water hidden inside. The famous ocean explorer Jacques Cousteau said that “it is funny that we called this planet Earth – there is so little of it”.
So, what makes this molecule – so “miraculous”?
Well, for one – it is polar.
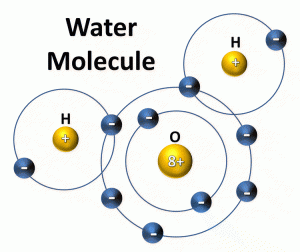
Image: Florida Atlantic University
A polar molecule is one that is electrically charged. To explain this, we will have to do a little bit of chemistry – hold on, we can do this. We know that elements are made up of positively charged protons, negatively charged electrons, a neutrally charged neutrons. We know the protons and neutrons are strongly bonded together in the nucleus and that the electrons orbit the nucleus in what are called energy shells. These energy shells are like planets orbiting the sun – sort of – you will learn more on how that all works in chemistry. We know that the atomic number of an element on a periodic table tells you how many protons and electrons the atom has in its neutral state. You may, or may not, know that there is a finite number of electrons that can occupy the orbiting energy shells. When an energy shell becomes full, any additional electrons must go into the next shell out from the nucleus. You may, or may not know, that within each energy shell there are subshells that allow two electrons to orbit – one clockwise, one counterclockwise – and that each subshell increases the number of orbital paths by an odd number. Maybe we are getting lost – so let’s SEE it.
Oxygen is atomic #8 on the periodic table. This means it has eight protons in the nucleus and eight electrons orbiting this nucleus when the atom is neutral. The first energy shell has ONE suborbital – the s suborbital. This s subshell has space for two electrons – one orbiting clockwise, one counterclockwise. So, two electrons can fit here – this leaves six more.
The second energy shell also has an s suborbital (which can hold two) but also has a p suborbital. If s has one orbit (which can hold two electrons), the p has three – a third energy shell would have an s, p, and a d subshells. The s has one orbit, the p three, and the d has five (each additional subshell increases by an odd number). So, the next out, f, would have how many suborbitals??? Seven – correct! Each of these can hold two electrons.
For oxygen we have the first energy shell which only has an s – so two are placed here – leaving six.
The second energy shell has an s and a p. Two can go into the 2nd s – leaving four and those four go into the p shell. The way these electrons fill the subshells is each gets one electron first, then we go back and add the second.
1st energy shell s – ↑↓ – one clockwise and one counterclockwise
2nd energy shell s ↑↓
P ↑↓ ↑_ ↑_ – one in each space first, then go back to fill them until they are full
So, Oxygen s – ↑↓
P ↑↓ ↑_ ↑_
Do you see how it fills each suborbital one at a time?
S will get one ↑ and then one ↓ and it is full – so can only take two.
P has three “spaces” each getting an ↑ first (so the first three will fill a space first) and then go back and place additional electrons in the ↓ rotation until it is full.
If you understand this, then you should understand that the first energy level can only hold two.
But the second energy level (with an s and a p) can hold eight.
What would the third energy look like?
S ___
P ___ ___ ___
D___ ___ ___ ___ ___ – remember each increases by an odd number.
So, what would the total number of electrons the 3rd energy level could hold? Answer: 18
To fill ALL 3 energy level the atom would need 28 electrons. 2 would go into the s of the first energy shell. 8 would go into the s and p of the second energy shell, and 18 would go into the s, p, and d of the third energy shell.
Here is another bit of information… each atom on the periodic chart MUST have its last s and p full. If they have enough electrons to need four energy levels – then the s and p of that energy level MUST be full. If not, will need to either CAPTURE electrons from some other atom – or RELEASE them to another.
In the case of oxygen, you will see it has 2 energy levels (shells) and the last s and p are NOT full. It needs TWO more to complete the p. Where on Earth is it going to find two electrons to fill the p?
Enter… hydrogen.
Hydrogen has the atomic number of 1. Meaning it has one proton and one electron. If we place this one electron in the orbiting shells you will see that it cannot even fill the first energy shell (which needs two).
Hydrogen 1s ↑_
It needs a second electron to fill its first energy level. (NOTE HERE: THE FIRST ENERGY LEVEL IS CONSIDERED FULL IF THE s IS FULL – IT DOES NOT NEED A p TO DO SO). So, hydrogen does not have to find enough electrons to fill a second energy level – it is good with just the two. But it does need a second one to meet that two limit.
Is oxygen going to give it an electron to hydrogen to fill its first shell? No… it is not going to give anything – it is looking for two new ones, not looking to give away any. If it did, then oxygen would now need THREE electrons to fill its last shell – will not do this.
Will hydrogen give its only electron to oxygen to partially fulfill the two-electron requirement oxygen has? No, that is the only electron hydrogen has – it will not give it to anybody. So, what are we going to do here?
We are going to share.
Hydrogen will allow its only electron to orbit one of the p subshells of the oxygen atom – but then the oxygen atom will provide one electron (in addition to the one hydrogen electron) to orbit the hydrogen atom giving it two electrons… for a few seconds and then they move back to the oxygen. So, back and forth, back and forth. This sharing of electrons forms what is called a covalent bond.
But this only covers ONE of the needed electrons that oxygen needs. So, we will need TWO hydrogens to pull this off… hence H2O.
You will also note that the location of the two hydrogens in next to each other the grand scheme of things.
Water s – ↑↓
P ↑↓ ↑↓H ↑↓H

Image: Florida Atlantic University
The oxygen atom is a larger atom and will hold the electrons of the hydrogen a bit longer than the hydrogen will hold them. With a greater number of electrons than protons in the oxygen atom (8 protons but 10 electrons when it is holding the two from hydrogen) there is a net negative charge to the oxygen atom (electrons are negative and they have more of them than positive protons). In the same sense – the lack of electrons in a hydrogen atom (1 proton, 0 electrons when oxygen has them) gives a net positive charge to the hydrogen atom. SO, the positive end of the water molecule (the hydrogen) is opposite the negative end (the oxygen) and the water molecule is electrically charged – IT IS POLAR.
So, what is the significance of all of this…
Most compounds we find in nature are what are called ionic compounds. In this case the electrons are not shared, but actually transferred from one atom to another. Let’s take the other big compound found in the ocean – salt.
There are many types of salts – sodium chloride (NaCl) is just one of them – but we will use NaCl for this example.
Sodium (Na) has the atomic number of 11 –
11 protons and 11 electrons – let’s use the energy shells and fill with these 11 electrons.
Sodium 1s – ↑↓
2s – ↑↓
2p – ↑↓ ↑↓ ↑↓
3s – ↑_ ___
3p – ___ ___ ___
3d – ___ ___ ___ ___ ___
You will notice that it takes three energy levels (shells) to accommodate the 11 electrons of sodium.
You will also notice that the LAST s and p are NOT full. It will take 7 more electrons to do this. (Remember, we only need the s and p filled – not the d).
Or sodium can GIVE AWAY the one electron in the third shell (3s) and then the last s and p (2s 2p) WILL be full. IS IT EASIER TO GIVE AWAY ONE, OR FIND SEVEN? Yes… atoms are lazy… they will give the one 3s away – but to whom?
Enter chlorine…
Chlorine is atomic number 17
Chlorine 1s – ↑↓
2s – ↑↓
2p – ↑↓ ↑↓ ↑↓
3s – ↑↓
3p – ↑↓ ↑↓ ↑_
3d – ___ ___ ___ ___ ___
Can you see what is going to happen here?
Chlorine needs ONE electron in that last space of 3p to fill the 3s 3p of its third shell.
Sodium GIVES it’s 3s electron to Chlorine – who places it in the last space of its 3p and now the 2s 2p of Na is full as is the 3p of Cl. They are both happy.
But now – Na has 11 protons and one only 10 electrons – protons win – the Na atom now becomes a positively charged ion (Na+). Chlorine now has 17 protons but 18 electrons – electrons win, and Cl becomes a negatively charged ion (Cl–). Opposites attract (+-) and sodium and chlorine form an ionic bond (not sharing, the electron is transferred). Na+Cl– = NaCl… salt.
Place this ionic compound (NaCl) into water – which is polar – and the positive side of the salt molecule (Na+) is attracted to the negative side of the water molecule (O–) while the negative part of salt (Cl–) is attracted to the positive side of water (H+) and the NaCl (salt) molecule is ripped apart – dissolved.
Again, most molecules and compounds in nature are ionic – and water will rip them apart. It is called the universal solvent. So, anything placed into water will eventually dissolve – metal, sand, glass, plastic, humans.
Humans…
Yea, what about that?
What about the fish and seaweed in the sea? Are THEY going to dissolve?
Nope… and here’s why… not EVERYTHING is dissolved by water. We have all heard oil and water do not mix. They do not. The type of compounds that include oils and fats are called lipids, and lipids do not dissolve in water. So, most of the material inside of a cell is water, but the cell membranes are lipids. So, you will not dissolve from the inside out. And a fish living in water has many lipids in its skin (as to do all creatures) – so you will not dissolve from the outside in…. perfect… what a planet.
So, why is this all important for life on the planet?
Well, we consume food – which we need for energy and nutrients. We eat large objects that are broken down by chewing and digested by enzymes and water. These elemental components of our food are then transported through the body to the cells where they are needed by water-based fluids. Toxic by-products of digestion are removed from the cells by water-based fluids and converted into urine where they are excreted from the body in… you guessed it… a water-based fluid (urine). Plants are no different. They do not consume their food the way we do but they do absorb compounds (fertilizers) from the soil where they have been broken down by microbes and water and absorbed by absorbing water into the roots where it is carried throughout the plant in a circulatory system based on… can you guess… first two guesses do not count… you’ve got it – water. How it is absorbed by the lipid covered cells of the plants and animals is a WHOLE NOTHER discussion – no time for that one now.
This miracle molecule is very abundant on our planet. 70% of the surface of the Earth is covered by it. But the distribution of usable forms of water is not evenly distributed. 90% of the water on the surface is seawater – which has dissolved ions in it. Remember how water dissolved the NaCl molecule? Well, the definition of a “salt” is the product of an acid and a base. Example:
HCl + NaOH à HOH + NaCl (HOH is H2O by the way). So, NaCl is a salt.
How about H2SO4 (sulfuric acid) + NaOH à HOH + NaSO4 (sodium sulfate – which is another type of salt)
Of course, in the presence of water these compounds would “dissolve into Na+, Cl–, SO4–. So, any of the compounds found in the water would disassociate into their ions and be in the water itself. These ions are electrically charged (Na+, Cl–, SO4–) and so provide an electric current. Measure the conductivity of the water (how much electric current it has) is one way of determining how many ions are dissolved in the water. The amount of dissolved ions in the water is what we call salinity. Most think of salinity as “how salty the water is” and – in a way – they are correct. Because of the dissolved ions in seawater are products of salts – sodium and chloride making up over 70% of the ions there – it looks and smell salty. Measuring the conductivity of the water with a meter that can detect electric conduction can give a value that can be converted into salinity. Most marine scientists today use an instrument that does this conversion for you and you just record salinity from it. Salinities in water usually run between 0 and 35. The unit of measure is part per thousand. We know that percent (%) is parts per hundred (such as the grade you will get on your next exam). But parts per thousand would use 2 zeros in the denominator (‰). So, we would record the salinity of, say Bayou Texar, as 17‰. You could also write 17 ppt.
The thing is life cannot use saline water for the life-giving processes we described above. You either have to find freshwater (0‰) or have a method of excreting salt out of the water to make it freshwater – this is how marine organisms do it. More on this later.
Image: USGS
By the way…
Since 90% of the water on the surface of the planet is seawater (and of no use to many living creatures), this means 10% is freshwater. 90% of that freshwater is locked up as ice at the north and south poles, leaving 10% of the freshwater (1% of the total surface water) available for life – and much of this is in the ground. So, with a planet abundant with water – getting enough good, clean drinking is not as easy as you think. Water is precious and should be conserved.
This is a lot.
But there is SO much more to the miracle molecule than what we have gone over. WE will learn more about it as we move through this course. Now it is WAY PASS time for an activity.
ACTIVITY
We are going to do our best to include the full scientific process we covered in THE NATURE OF SCIENCE in each activity we do from here on out. In this activity we are going to observe and record what happens to plants that do not get their needed amount of water. The idea is to NOT provide water to a series of plants for one week and record what happens. We are not intending for them to die – so if they look bad mid-week (LOE) – give them water and call that the end point.
We can set it up as a series.
– The hypothesis is that plants will need a significant amount of water over the course of a week to survive.
– Our independent variable is the amount of water we add each day.
– Our dependent variable is how the plant looks/reacts to this amount.
– Remember our constants – WHAT ELSE COULD CAUSE A PLANT TO LOOK BAD BESIDES NOT ADDING WATER. Make a list of these and DO YOUR BEST to keep them constant throughout the experiment.
– (n=?) How many plants are you going to test? This will be up to your parents – DON’T KILL ALL OF THEIR PLANTS 😊 See what they are allowing you to experiment with. You could do something like this…
Ø Have three plants you add one cup of water to each day
Ø Have three plants you add two cups of water to each day
Ø Have three plants you add three cups of water to each day
Ø Etc.
Ø THEN THERE IS THE CONTROL – “NO TREATMENT” – three plants where no water is added all week.
In this scenario above n=12. Again, we know that in “the real world” n=1000 or more, but we get the idea.
KIDS ACTIVITY
Here are couple of things the kids can do that could be fun.
1) Place some seawater in a cup and let it stand in the sun. As the sun evaporates the water, the ions are allowed to re-bond and salt will re-form. They should see this pretty quickly. NOTE: MANY OF THE LOCAL WATERWAYS ARE POLLUTED FROM THE RUN-OFF OF HURRICANE SALLY. YOU MIGHT WANT TO GET THIS FROM A SALTWATER TANK IF YOU HAVE ONE.
2) You do not have to do the complete experiment from above – but you could stop watering a plant for a couple of days – record what happens – and then begin watering again – record what changes happen this time.
BE SURE TO DRINK PLENTY OF WATER EVERY DAY!!!
 0
0
