Jacques Cousteau once said, “it is strange that they call this planet Earth… there is so little of it”. This is true. 70% of the surface of this planet is covered in water but to call it that would be weird. Memorizing the planets in their order from the sun… Mercury… Venus… Water… Mars… Jupiter… it just does not work. Maybe planet “Aqua”? Nope… it has to be Earth… sound like home.

Photo: Molly O’Connor
But that does not erase the fact that the surface of the planet is mostly water. It is found in rocks and minerals, beneath the surface of the Earth, makes up 90% of our cells and our bodies, runs through all plants and trees, is vapor in the air, it is everywhere and it is because it is everywhere that life can exist here.
People have been known to go up to 70 days without food. At the end of that period they probably do not feel great and are not up for a game of tennis, but they are still alive. Most humans cannot go more than three days without water. We know we must drink several glasses of it each day. A lack of water can cause organ problems and more. Tom Hanks’ character in Castaway drank basically only water after he returned from living on that island for four years struggling to find it. It is a miracle compound.
After the Civil War Lt. Colonel John Wesley Powell made several expeditions to the American west and became the first to navigate the Colorado River through the Grand Canyon. He mentioned in his reports that this area of our new country would be difficult to settle because of the lack of water. But that Florida was so wet, it would never have to worry about that.
Isn’t that weird…
Here we are in the 21st century discussing water quality and quantity in a state like Florida. But those discussions are happening, and the issues are real. For a planet so full of water we could actually have wars over it may not be too science fiction. We need it.
This will be the focus of these lessons on water – quantity and quality – and how we can conserve this MUCH needed resource for our crops, livestock, pets, and ourselves. But let’s first look at this magical compound we call water.
Most of us know that is two parts hydrogen, one-part oxygen – H2O.
Those who know more chemistry will understand, by using the Lewis-Dot formula method, why the hydrogens attach to the oxygen the way they do. The two hydrogens sit on top of the oxygens head – looking like Mickey Mouse ears. This arrangement, and the time electrons circle both elements in this covalent compound, make the molecule polar… magnetic. It is like the ole Brio Trains of our youth. The positive red of one train car connects the negative green of another. If you try to push a red magnetic of one car to the red of another – they will repel each other… opposites attract.
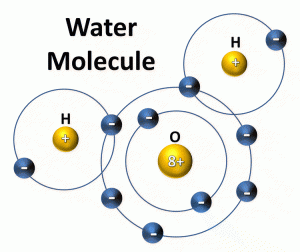
Image: Florida Atlantic University
The polarity of water causes it to bond with other water molecules. The hydrogen “Mickey ears” being the positive end of the molecule and the oxygen end being negative. The hydrogen end of one water molecule will be attracted to the oxygen end of another and the molecules bond together like magnets.
ACTIVITY 1
My plan was to do all activities outside, but I think this inside one would be good here. We are going to do a little activity for you to better understand the polarity of water.
This works best if you have some food coloring – I did not so the photos may not be the best.
If you can find a bowl with a flat bottom (as pictured) and a ¼ teaspoon (as pictured) you can set this up.
The idea is to place 4 ¼ quarter teaspoon drops of water in different locations at the bottom of the bowl.
If the bottom of the bowl is flat, and you were careful not to mix, you should have 4 separate pools of water.
Now use the teaspoon to mix the water pools into one pool.
They should mix and form one larger pool of water.
Now use the teaspoon to separate the large pool back into 4 small pools.
You will find this cannot happen. The molecules are now joined due to the polarity of the water. It is true that if you applied enough energy you could separate them – just as you could separate two magnets if you pull hard enough.
If you repeated this using a ¼ teaspoon of sand (do it!), you will find that you could mix the four “pools” together, but that you could (almost) recreate the original four pools. Sand molecules do not attract each other as water molecules do.



Here is a video that can help demonstrate the polarity of water
You would think the bonding of water molecules would make it difficult to swim through… and it does. Walking 100 feet through air is much easier than walking 100 feet through water. Water is certainly denser than air but there is energy needed to separate the hydrogen bonds holding water together that fish and crabs must provide in order to move forward. Fish are designed in a fusiform (bullet-shape) manner to move through this medium in a more efficient manner. Marine birds, reptiles, and mammals are also more “fish-looking” when they are swimming through the water. This shape works well.
ACTIVITY 2
It was supposed to rain in town last night. Take a walk around your yard and see where water was captured so that the animals in your yard can obtain it. How do you think the plants in your yard got water after last nights rain? If you cannot find any water in your yard – where do you think it went?
 0
0
