Paul Maneval1*, Charles A. Jacoby2, Holden E. Harris3, Thomas K. Frazer4
Motivation
Historically, the staghorn coral Acropora cervicornis was a dominant foundation species that provided much of the rugosity and structural complexity on Caribbean coral reefs. However, the abundance of staghorn corals has declined substantially throughout the Caribbean, with some areas losing more than 97% of historical cover over the past five decades. The ability to effectively and efficiently scale operations to restore past losses and keep pace with continuing declines remains a key challenge. For example, many efforts employ PVC “trees” deployed in relatively shallow water; however, recent results from Puerto Rico indicated that a vertical frame deployed in deeper water could yield higher growth rates and reduced maintenance compared to existing practices.

Objectives
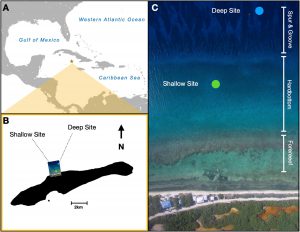
Given the impetus to scale up production to augment limited natural recovery, managers and researchers should consider how the design and location of the nurseries affect the growth of different genotypes of corals and the effort required for maintenance. To elucidate such influences, we grew fragments of different genotypes (5 varieties) on differing structures (trees and frames) at two depths (6–8 m and 16–18 m). Total linear extensions, accumulation of biofouling, and cleaning time were measured over 198 d to assess the growth of fragments and the effort required to maintain nurseries.

Findings overview
We found significantly higher mean daily incremental growth rates for fragments of the yellow genotype (24% higher than the next fastest-growing genotype and 74% higher than the slowest growing genotype), fragments held at the deep site (36% higher than fragments held at the shallow site), and fragments held on frames (13% higher than fragments on trees). If linear growth continued, the estimated mean annual growth for a fragment of the fastest-growing genotype held on a frame in deeper water would be 88 cm y-1, which was over 2x faster growth rate than a fragment of the slowest growing genotype held on a tree in shallow water. Temperature data suggested that nurseries at the shallow site were exposed to potentially stressful sea surface temperatures during warmer months, which correlated with a 3.5× higher rate of accumulation of algal biomass at the shallow site. If linear growth continued, the estimated mean annual growth for a fragment of the fastest-growing genotype held on a frame in deeper water would be 88 cm y-1, which was over 2× faster growth rate than a fragment of the slowest growing genotype held on a tree in shallow water. Collectively, the results demonstrated that improved production and reduced maintenance can result from considering the genotype of fragments to be cultured and the design and location of nurseries to support the restoration of coral reefs.
1 School of Natural Resources and Environment, University of Florida, Gainesville, FL, USA.
2 Soil and Water Sciences Department, University of Florida, Gainesville, FL, USA.
3 Nature Coast Biological Station, Institute of Food and Agriculture Sciences, University of Florida, Cedar Key, FL, USA
4 College of Marine Science, University of South Florida, St. Petersburg, FL USA.
Full Article: https://www.frontiersin.org/articles/10.3389/fmars.2021.670474/abstract
*Correspondence:
Paul Maneval
paul.maneval@noaa.gov
 0
0
