Have you noticed some white buildups on dishes after you run the dishwasher? Do your faucets have a crusty sediment on them? Highly likely it is because your water hard.

What is hard water?
Hard water comes from calcium and magnesium that are naturally found in the underground rock layers of limestone where we withdraw groundwater. Hard water is not harmful for human consumption. From a health standpoint, calcium and magnesium have no adverse effects. In fact, they are essential daily minerals that our bodies need. However, when calcium and magnesium permeate water, they build up on contact surfaces, such as pipes and water heaters, and decrease the effectiveness of soaps and detergents.
How hard is our water?
The hardness of water relates to the amount of calcium and magnesium in the water. The more minerals present, the harder the water is. Hardness is expressed in one of two units of measurement: parts per million (ppm) or grains per gallon (GPG) of calcium and magnesium dissolved in water. One ppm means that one unit of calcium carbonate is dissolved in one million units of water. When these minerals in water are higher than 120 ppm or 7.0 GPG, the water is considered hard by drinking water standards. Table 1 shows the degree of hardness standard as established by the American Society of Agricultural Engineers (S-339) and the Water Quality Association.
Table 1. Water Hardness Classification
| Degree of Hardness | Parts Per Million (ppm) | Grains Per Gallon (GPG) |
| Soft | <17.0 | <1.0 |
| Slightly Hard | 17 – 60 | 1.0 – 3.5 |
| Moderately Hard | 60 – 120 | 3.5 – 7.0 |
| Hard | 120 – 180 | 7.0 – 10.5 |
| Very Hard | >180 | >10.5 |
The hardness of water in Marion County is around 180 ppm or 10.5 GPG. Yes, our water is “Hard”, but not “Very Hard”. When the hardness is higher than 200 ppm, water treatment plants usually soften the water if you get your drinking water from a local utility instead of a private well.
How to soften the water?
To soften or not to soften? It is a personal preference. If you choose to remove the hardness (i.e., calcium and magnesium) from your water, a traditional ion exchange unit is the most effective way to do so. It is often referred to as a water softener. In these units, the negatively charged calcium and magnesium ions in the water are exchanged with the positively charged ions, typically sodium, as shown in Figure 2.
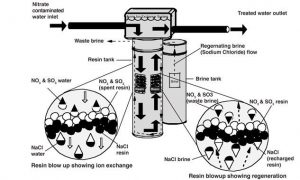
Keep in mind, to remove one unit of hardness, you are adding about half a unit of sodium into the water. You won’t taste the sodium as the added amount is not too much, but it is too much for someone who is on a low sodium diet. There are some new water softeners that use potassium instead of sodium. The ion exchange process is the same, but with the addition of potassium instead of sodium to the water. Talk to your doctor if you have a kidney problem before drinking potassium-softened water. Instead of a whole-house water softening system, you can also install a by-pass system. In that way, the hot water line will be softened but cold water will not. Then you won’t be drinking added sodium or potassium.
Recently there are some non-traditional softening units using magnets, electronic charges, or citric acid to remove hardness, but they have shown mixed results of effectiveness and are not generally recommended.
 2
2
