Recently there was a report of high fecal bacteria in a portion of Perdido Bay. I received a few concerned emails about the possible source. Follow up sampling from several agencies in both Florida and Alabama confirmed the bacteria was there, the levels were below both federal and state guidelines (so no advisory issued), and a small algal bloom was also found. It was thought the cause was excessive nutrients and lack of rain.
We hear this a lot.
Excessive nutrients and poor water quality.
What is the connection?
Many people understand the connection, others understand some of it, others still do not understand it. UF IFAS has a program called LAKEWATCH where citizen science volunteers monitor nutrients in some of lakes and estuaries within the state. Here in Escambia County, we have six such volunteers. Some of the six bodies of water have been monitored for many years, others are just starting now, but the data we have shows some interesting issues – many have problems with nitrogen. Let’s look closer.
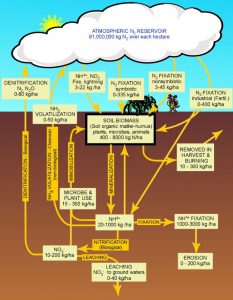
Image: UF IFAS
We have all heard of nitrogen. Many will remember from school that it makes up 78% of the air we breathe. In the atmosphere nitrogen is present as a gas (N2). It is very common element found in living creatures – as a matter of fact, we need it. Nitrogen is used to build amnio acids – which builds proteins – which is needed to produce tissue, bone, blood, and more. it is one of the elements found in our DNA and can be used to produce energy. However, it cannot do these in the atmospheric gas form (N2) – it needs to be “converted” or “fixed”.
One method of conversion is the weather. Nitrogen gas are two molecules of nitrogen held together by strong chemical bonds (N2). However, lightning provides enough energy to separate N2 and oxidize it with oxygen in the atmosphere forming nitrogen dioxide (NO2). This NO2 combines with water in the atmosphere to form nitric acid (HNO3). Which can form nitrates (NO3) with the release of the hydrogen and nitrate (NO3) is usable by plants as a fertilizer… a needed nutrient.
But much of the usable nitrates do not come from the atmospheric “fixing” of nitrogen via lightning. It comes from biological “fixing” from microbes. Atmospheric nitrogen can be “fixed” into ammonia (NH3) by bacteria. Another group of bacteria can convert ammonia into nitrite (NO2), and a third group can convert it from nitrite to the usable form we know as nitrate (NO3). Ammonia can also be found in the environment as a waste product of life. As we use nitrogen within bodies it can be converted into ammonia – which can be toxic to us. Our bodies remove this ammonia via urine, and many times we can smell this when we go to the restroom. Nitrogen fixing bacteria can convert this ammonia to nitrites as well and complete the nitrogen cycle.
Once nitrogen has been fixed to the usable nitrate it can be taken up by plants and used within. Animals obtain their needed nitrogen by eating the plants or eating the animals that ate the plants. In both cases, nitrogen is used for protein synthesis in our bodies and unused nitrogen is released into the environment to continue the cycle.
So, what is the connection to water quality?
You might think that excessive nitrogen (nutrients) in the water would be a good thing. Released ammonia, though toxic, could be “fixed” into nitrite and eventually nitrate and recycled back into life. And you would right. Excessive amounts of ammonia though, may not be converted quick enough and a toxic state could occur. We see this in aquaculture ponds and home aquaria a lot.

Photo: University of South Florida
But what about excessive nitrates? Shouldn’t that be good for the plants?
The concept makes sense, but what we see with increase plant growth in aquatic systems is problematic. Excessive plant growth can cause several problems.
1) Too much plant growth at the surface (algae, leafy vegetated material) can block sunlight to the other plants living on the bottom of the waterway. This can cause die off of those plants and a mucky bottom – but there is more.
2) Excessive plant growth at the surface and the middle of the water column can slow water flow. Reduced water flow can negatively impact feeding and reproductive methods for some members of the community, cause stagnation, and decrease dissolved oxygen – but there is more.
3) Plants produce oxygen, which is a good thing, so more plants are better right? Well, they do produce oxygen when the sun is up. When it sets, they begin to respirate just as the animals do. Here excessive plants can remove large amounts of dissolved oxygen (DO) in the water column at night. If the DO levels reach 3.0 µg/L many aquatic organisms begin to stress. We say the water is hypoxic (oxygen starving). When a system becomes hypoxic the animals will (a) come to the surface gasping, (b) some even approach the beach (the famous crab jubilee of Mobile Bay), (c) leave the water body for more open water, (d) die (a fish kill). This is in fact what we call a dead zone. Not always is everything dead, in many cases there is not much alive left – they have moved elsewhere so we say the bottom is “dead”. Here is something else… as the dead fish and (eventually) dead plants settle to the bottom they are decomposed by bacteria. This decomposition process requires dissolved oxygen – you guessed it – the DO drops even further enhancing the problem. In some cases, the DO may drop to 0.0 µg/L. We say the water is now anoxic (NO oxygen). I have only seen this twice. Once in Mobile Bay, and once in Bayou Texar. But I am sure it happens more often. The local environment can enhance (or even cause) this problem as well. Warm water holds less oxygen and much of the oxygen dissolved in water comes from the atmosphere – by way of wave action. So, on hot summer days when the wind is not moving much, and excessive nutrients (nitrogen) is entering the water, you have the perfect storm for a DO problem and possible fish kill.
4) Oh, and there is one other issue… some of the algae that produces these blooms release toxins into the water as a defense. These are known as harmful algal blooms (HABs). Red tide is one of the more famous ones, but blue-green blooms are becoming more familiar. So now you have a possible hypoxic situation with additional toxins in the water that can trigger large fish kills. Some of these HABs situations have killed marine mammals and sea turtles as well.
Though this process can occur naturally (and does) excessive nutrients certainly enhance them, and in some cases, initiate them. So, too much nitrogen in the system can be bad.
So, what does the LAKEWATCH data tell us about the Pensacola Bay system?
Well, first, we have not had volunteers on all bodies of water for the same amount of time. We currently have volunteers monitoring (1) northern Pensacola Bay, (2) Bayou Texar, (3) Bayou Chico, (4) Bayou Grande, (5) Big Lagoon, and (6) lower Perdido Bay. Pensacola Bay has JUST started, and Big Lagoon has not even started yet (COVID-19 issues) – so we only have data from the other four. Bayou Texar has the longest sample period at 13 years.

Second, this program does not sample for just nitrogen, but another key nutrient as well – phosphorus. When you look at a bag of fertilizer you will see a series of numbers looking like: 30-28-14. This would be nitrogen, phosphorus, and potassium. People adding fertilizer to their lawns should know which nutrient they need the most and can by a fertilizer with a numerical concentration that is best for their lawn. You can have your soil tested at the county extension office. But the point here is that there is more than nitrogen to look at and, as we have learned, more than one form of nitrogen out there. So, what we do at LAKEWATCH is monitor for total nitrogen (TN) and total phosphorus (TP).
Another parameter monitored is total chlorophyll a (TC). The idea is… if there are excessive amounts of nutrients in the water there will be excessive amounts of algae. You could collect a sample of water and count the number of algal cells in the water – but another way is to measure the amount of chlorophyll in the water as a proxy for the amount of algae. Chlorophyll, of course, is the compound within plants that allows photosynthesis to happen. There is a chemical process used to release the chlorophyll within the cells and you can then use an instrument to measure the amount of chlorophyll in the water.
LAKEWATCH volunteers also monitor water clarity. It is true that clarity can be impacted by sediments in the water as much as an algal bloom, but anything that contributes to less sunlight reaching the bottom can be problematic for some bodies of water. This is done by lowering a disk into the water and measuring the depth at which it “disappears”.
For those not familiar with the term salinity, it is the measure of the amount of dissolved solids in the water – what most people say, “how salty is it?”. For reference, the Gulf of Mexico is usually around
35‰, most open estuaries are between 20-30‰.
Below is a table of the LAKEWATCH data we have as of the spring 2020.
| Year of Sampling | Body of Water | Total Phosphorus (µg/L) | Total Nitrogen (µg/L) | Total Chlorophyll (µg/L) | Water Clarity (feet) | Salinity (‰) |
| 2014 – 2018 | Bayou Chico | 20-30 | 350 – 600 | 10 – 30 | 2.6 – 4.2 | 7.0 – 8.2 |
| 2012 – 2017 | Bayou Grande | 16 – 19 | 320 – 340 | 5 -6 | 4.0 – 5.2 | 17 – 18 |
| 2007 – 2018 | Bayou Texar | 17 – 18 | 600 – 800 | 6 – 8 | 3.4 – 3.8 | 8 – 10 |
| 2014 – 2018 | Lower Perdido | 15 -16 | 350 – 360 | 5 -6 | 5.3 – 6.1 | 13 -14 |
| STATE AVG. | (includes lakes) | 25.0 | 309 | 3.7 |
There are a couple of things that stand out right away
(keep in mind some water bodies have not been monitored very long by LAKEWATCH).
1) Phosphorus is not as big a problem in our part of the state. In the peninsula part of Florida there is a lot of phosphorus in the sediments and much of it is mined. You can see this in the average value for the state. Actually, because of this, many of the central and south Florida lakes are naturally high in phosphorus and this is not considered “polluted”. All that said, there are higher levels of phosphorus in Bayou Chico. Which is interesting. More on solutions in a moment.
2) We have a lot of nitrogen in our waters. Bayou Texar in particular is much higher than the state average. More on this in a moment.
3) We have a little more chlorophyll than the state average, but not alarming.
4) Bayou Grande and lower Perdido are clearer than Bayou’s Texar and Chico.
5) All these bodies of water are less than 20‰. More on this as well.
So, comments…
1) We already discussed the phosphorus issue (or non-issue), but what about Bayou Chico? Phosphorus is NOT introduced to the system from the atmosphere as nitrogen is – rather, it comes from the sediments. High levels of TP would suggest high levels of sediments in the water column (the water clarity data supports this) – which suggest high levels of run-off. The watershed for Bayou Chico is highly urbanized and run-off has historically been a problem.
2) Nitrogen can come from many sources, but when numbers get high – many will hypothesize they are most likely from lawn run-off (fertilizers), or sewage (septic leakage, sanitary sewage overflows, animal waste). There are certainly other possibilities, but this is where most resource managers and agencies begin.
3) Elevated chlorophyll indicates elevated primary production. This is not unusual for an estuary. They are known for their high productivity. Bayou Chico seems have more algae than the others. Most probably due to the increase levels of nutrients entering the watershed.
4) Bayou Grande and lower Perdido Bay have better water clarity than Bayou’s Chico and Texar. Though all four bodies of water have significant coastal and watershed development, Bayou’s Chico and Texar and completely developed as well as their “feeder creeks”. Again, indication of a run-off problem.
5) All four bodies of water have several sources of freshwater input as well as stormwater run-off that has contributed to the lower salinities found here. It is possible that the salinities here were less than 20‰ prior to heavy development.
Possible Solutions….
There is a common theme with each of these – stormwater run-off. Rain that historically fell on the land and percolated into the ground water, now flows off impervious surfaces (streets, driveways, parking lots, even buildings) into drainage pipes and discharges into the waterways. This stormwater carries with it much more than just fertilizers and animal waste, it carries pesticides, oil, grease, solid waste, leaf litter, and much more.
How do you reduce stormwater?
Well, there is not much you can do with impervious surfaces now, but the community should consider alternative materials and plans for future development – what we call “Green Infrastructure”. Green roofs, pervious streets and parking lots, there are a lot of methods that have been developed to help reduce this problem.
Another consideration is Florida Friendly Landscaping. This is landscaping with native plants that require little (or no) water and fertilizer. It also includes plants that can slow run-off and capture nutrients before they reach the waterways and methods of trapping run-off onto your property.
If you happen to live along a waterway, you might consider landscaping your property by restoring some of the natural vegetation along the shoreline – what we call a living shoreline. Studies have shown that these coastal plants can remove a significant amount of nitrogen from the run-off of your property as well as reduce coastal erosion and enhance fisheries by providing habitat.

What about sewage issues?
Unfortunately, most septic systems were not designed to remove nitrogen – so leaks occur and will continue. The only options you have there are (a) maintain your septic by pumping once every five years, (b) consider taping into a nearby sewage line.
Sewers systems are not without their problems. Sanitary sewage overflows do occur and can increase nitrogen in waterways. These are usually caused by cracks in old lines (which need to be replaced), are because we flush things down drains that eventually “clog the arties” and cause overflows. Things such as “flushable wipes”, which are flushable – they go down the drains – but they do not breakdown as toilet paper does and clog lines. Cooking grease and oil, and even milk have been known to clog systems.
Our LAKEWATCH volunteers will continue to sample three stations in each of their bodies of water. We are looking for a volunteer to monitor Escambia Bay. If interested contact me.
If you are interested learning more about green infrastructure, Florida Friendly Landscaping, or living shorelines lines contact your county extension office (850-475-5230 for Escambia County). If interested in issues concerning sanitary sewage overflows or septic issues, contact your county extension office, or (if in Escambia or Santa Rosa counties) visit ECUAs FOG website (Fats, Oils, and Grease) https://ecua.fl.gov/live-green/fats-oils-grease.
References
The Weather Guys. 2018. Does Lightning Add Nitrogen to the Soil? University of Wisconsin-Madison.
https://wxguys.ssec.wisc.edu/2018/07/09/lightning/.
 0
0
